RESEARCH ON NEW DRUG DELIVERY SYSTEMS
The aim of the research of the pharmaceutical technology unit is to develop new delivery systems of drugs, peptides, nucleic acids and antigens.
The current research themes are :
Our research aims at enhancing and expanding to range of transdermal delivery of drugs, peptides as well as to optimize DNA delivery in the skin as alternatiev to injection.
We focussed in electrically enhanced transdermal drug delivery. Both iontophoresis (low intensity current) and electroporation (high voltage pulses) were shown to enhance transdermal drug delivery by several orders of magnitude (1, 2). Iontophoresis acts on the drug whereas electroporation increases skin permeability. Both induce negligible damage to the skin (3, 4).
Iontophoresis can be also used to extract endogeneus substances.
Reverse iontophoresis is currently investigated for the non-invasive monitoring of renal function.
Skin electroporation strongly enhances gene expression after intradermal at to a --- extend after topical delivery. Different applications of DNA electrotransfert are being developped (5).
Cutaneous microdialysis is used to follow free concentration in the dermis of a drug delivered topically or transdermally (6).
Members of the team : V. Préat (group leader), L. Daugimont, G. Vandermeulen, V. Wascotte (PhD Students)
Selected publications
- Jadoul
A., Mesens J., Caers W., de Beukelaar F., Crabbé R.,
Préat V.
Transdermal permeation of alniditan by iontophoresis: in vitro optimization and human pharmacokinetic data, Pharmaceutical Research, 13:1348-1353, 1996 - Vanbever
R., Lecouturier N., Préat V.
Transdermal delivery of metoprolol by electroporation, Pharmaceutical Research, 11:1657-1662, 1994 - Jadoul
A., Bouwstra J., Préat V.
Effects of iontophoresis and electroporation on the stratum corneum. Review of the biophysical studies, Advanced Drug Delivery Reviews , 35:89-105, 1999 - Denet
A-R, Vanbever R., Préat V.
Skin electroporation for transdermal and topical delivery, Advanced Drug Delivery Reviews, 56:659-674 - Pasvlej
N.,
Préat V.
DNA electrotransfer into the skin using a combination of one high- and one low-voltage pulse, Journal of Controlled Release, 106:407-415, 2005 - Mathy
F.X., Ntivunwa D., Verbeeck R.K., Préat
V.
Fluconazole distribution in rat dermis following intravenous and topical application: a microdialysis study, Journal ofPharmaceutical Sciences, 94:770-780, 2005
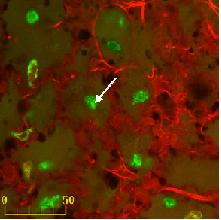
GFP
expression in the skin after DNA plasmid delivery
by electrotransfert
from Pavselj et al, 2005
[top]
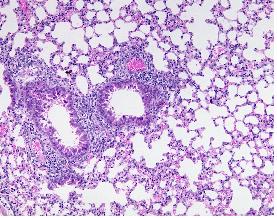
Our research team aims at optimizing inhalation aerosols for systemic delivery of therapeutic peptides and proteins and for local administration of vaccines as alternative to injection.
We optimize systemic absorption of therapeutic peptides and proteins from the lung (1) by i) designing dry powder aerosols with elevated deep lung deposition (2), ii) selecting appropriate excipients (3), iii) understanding the biological losses encountered by inhaled macromolecules in the lung. In this regard, we demonstrated that a primary source of elimination of macromolecules following delivery to the lung and prior to absorption into the bloodstream owed to clearance by alveolar macrophages (4).
We study the potential of inhalation aerosols for administration of vaccines (5). We showed that, according to the antigen or DNA vaccine molecule, the pulmonary route produced stronger or equivalent humoral and cellular responses systemically and locally in the lung as compared to injection. We currently optimize immunization efficacy by designing dry powder aerosols with an appropriate deposition within the lung. In addition, we use for their preparation GRAS excipients that have potential vaccine adjuvant properties. We recently demonstrated that the deeper the deposition of the vaccine molecule within the lung, the stronger the immune response.
Members of the team : R. Vanbever (group leader), N. Grancher (post-doctoral fellow), J. Ducreux, A. Minne (PhD students)
Selected publications
- Vanbever
R.
Performance-driven, pulmonary delivery of systemically acting drug, Drug discovery today: technologies, 2:39-46, 2005 - Bosquillon
C.., Lombry C., Préat V., Vanbever R.
Influence of formulation excipients and physical characteristics of inhalation dry powders on their aerosolization performance, Journal of Controlled Release, 70:329-339, 2001 - Codrons
V., Vanderbist F., Verbeeck R.K., Arras M., Lison D.,
Preat V., Vanbever R.
Impact of formulation and methods of pumonary delivery on absorption of parathyroid hormone (1-34) from rat lungs, Journal of Pharmaceutical Sciences, 93:1241-1252, 2004 - Lombry
C., Edwards D.E., Préat V. , Vanbever R.
Alveolar macrophages are a primary barrier to pulmonary absorption of macromolecules, American Journal of Physiology - Lung Cellular and Molecular Physiology, 286:L1002-L1008, 2004 - Lombry
C., Marteleur A., Arras M., Lison D., Louahed J.,
Renauld J-C, Préat V., Vanbever R.
Local and systemic immune responses to intratracheal instillation of antigen and DNA vaccines in mice, Pharmaceutical Research, 21:127-135, 2004
|
|
|
[top]
On the basis of our previous expertise in the preparation of polymeric biodegradable microspheres and nanoparticles for the oral delivery of vaccine and therapeutic peptides is undergoing (1,2).
The efficacy of the passage of those nanoparticles (4) with or without M cells targeting is evaluated in vitro and in vivo(5).
Nanoparticles in quaternized polycaprolactone are inviestigated for non viral gene delivery.
Members
of the team :
V. Préat
(group leader), M. Garinot (post-doctoral fellow)
F. Danhier A. des Rieux, V. Fievez,
F. Mathot, B.Vroman (PhD students)
Selected Publications
- Ould-Ouali
L., Ariën A., Rosenblatt J., Nathan
A., Twaddle P., Matalenas T., Borgia
M., Arnold S., Préat V.
Biodegradable self-assembling PEG-copolymer as vehicle for poorly water-soluble drugs, Pharmaceutical Research, 21:1581-1590, 2004 - Ould-Ouali
L., Noppe M., Langlois X., Willems
B., Te Riele P., Timmerman P., Brewster
M., Ariën A. , Préat V.
Self-assembling PEG-p(CL-co-TMC) copolymers for oral delivery of poorly water-soluble drugs: a case study with
risperidone, Journal of Controlled Release, 102:657-668, 2005 - Mathot
F., Van Beijsterveldt L., Préat
V., Brewster M., Arien A.
Intestinal uptake and biodistribution of novel polymeric micelles after oral administrationJournal of Controlled Release, 111:47-55, 2006 - Lemoine
D., Wauters F., Bouchendhomme S.,
Préat V.
Preparation and characterization of alginate microspheres containing a model antigen, International Journal of Pharmaceutics, 176:9-19, 1998 - des
Rieux A., Ragnarsson E., Gullberg E.,
Preat V., Schneider Y.J., Artursson
P.
Transport of nanoparticles across an in vitro model of the human intestinal follicle associated epithelium, European Journal of Pharmaceutical Sciences, 25: 455-46, 2005
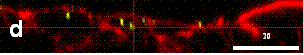
Nanoparticles
transport across an in vitro model of M-line
intestinal cells
from des Rieux et al, 2005
[top]
- Harvard University, Division of Engineering and Applied Science, Boston, USA
- University of Southern California, Keck School of Medicine, Pulmonary Research Center, Los Angeles, USA
- University of Berlin, Department of Pediatric Pneumology and Immunology, Allemagne
- University of Dundee, Division of Cell Biology and Immunology, United Kingdom
- Université catholique de Louvain, Unité de pharmacocinétique, métabolisme, nutrition et toxicologie, Belgique
- Université catholique de Louvain, Unité de pharmacologie cellulaire et moléculaire, Belgique
- Université catholique de Louvain, Laboratoire de pharmacothérapie, Belgique
- Université catholique de Louvain, Unité de Biochimie cellulaire, Belgique
- Cliniques universitaires Saint-Luc, Unité de Biochimie médicale, Belgique
- Université catholique de Louvain, Unité de Chimie médicinale, Belgique
- Université catholique de Louvain, Unité de Résonance magnétique biomédicale, Belgique
- Université libre de Bruxelles, Belgique
- Université de Liège, Belgique
- University of Bath, United Kingdom
- Université de Paris V, France
- Institut Gustave Roussy, France
- Université de Ljubljana, Slovénie